CervicalScreen Norway: Advancing cervical cancer prevention through innovation and research

CervicalScreen Norway (Livmorhalsprogrammet) is managed by the Cancer Registry of Norway, which operates under the Norwegian Institute of Public Health. It offers screening to women aged 25 to 69, providing an organised and accessible approach to detection of premalignant cervical lesions and prevention of cervical cancer development.
Program structure and objectives
Established in 1995, CervicalScreen Norway continuously evolves to optimise screening effectiveness within available resources. The program involves four key stakeholder groups:
- – Women invited to participate: The primary focus and target group of the program.
- – Healthcare providers: GPs, gynaecologists and some midwives who collect samples and manage follow-up visits.
- – Laboratories: Facilities that analyse samples and provide follow-up recommendations to the sample taker.
- – The administrative unit at the Cancer Registry: Responsible for reminders, data management, and program coordination.
The screening program is governed by a professional advisory group established by the Norwegian Directorate of Health, consisting of representatives from all regional health authorities.
The program aims to reduce cervical cancer incidence and mortality through a systematic, quality-assured, population-based screening approach. With a current participation rate of around 73%, CervicalScreen Norway strives to exceed 80%, while minimising unnecessary procedures, ensuring both clinical and economic efficiency.
Several steps have been undertaken to minimise unnecessary procedures and maximise the accuracy of the screening, ensuring both clinical and economic efficiency. Recognising that research-based improvement is a continuous process, a dedicated team of researchers systematically evaluates screening methods and identifies opportunities for further optimisation.
How CervicalScreen Norway works

- – Reminder by digital mail: Women in the target age group receive a reminder letter from the Cancer Registry to take a cervical screening test. The screening interval is five years, as all women now receive an HPV test and are recommended to undergo a new screening after five years.
- – Booking an appointment: Women schedule an appointment with their general practitioner (GP) or, in certain cases, a gynaecologist or midwife.
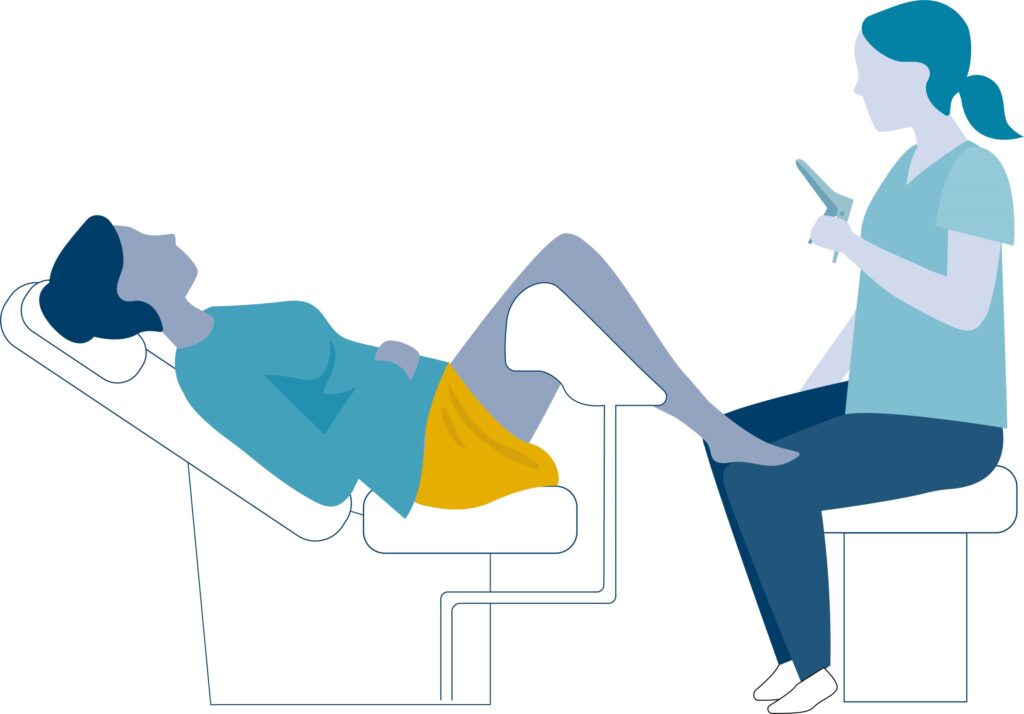
- – Sample collection and testing: A cervical sample is collected using a small brush and tested for human papillomavirus (HPV). If HPV is detected, cytology is used as a triage test. The women are then stratified by risk and will either be asked to undergo a new test after 1 or 3 years or undergo further examination, which includes a pelvic examination and colposcopy, with a biopsy performed if histologic confirmation is needed.
Before July 1, 2023, CervicalScreen Norway used cytology as the primary screening test for detecting cervical cell changes. Since then, the program has adopted HPV testing for the entire target population, aligning with the latest research demonstrating HPV testing’s higher sensitivity in detecting pre-cancerous changes. In January 2024, it was decided to implement extended genotyping in the risk-based algorithms.
Expanding access with HPV self-sampling
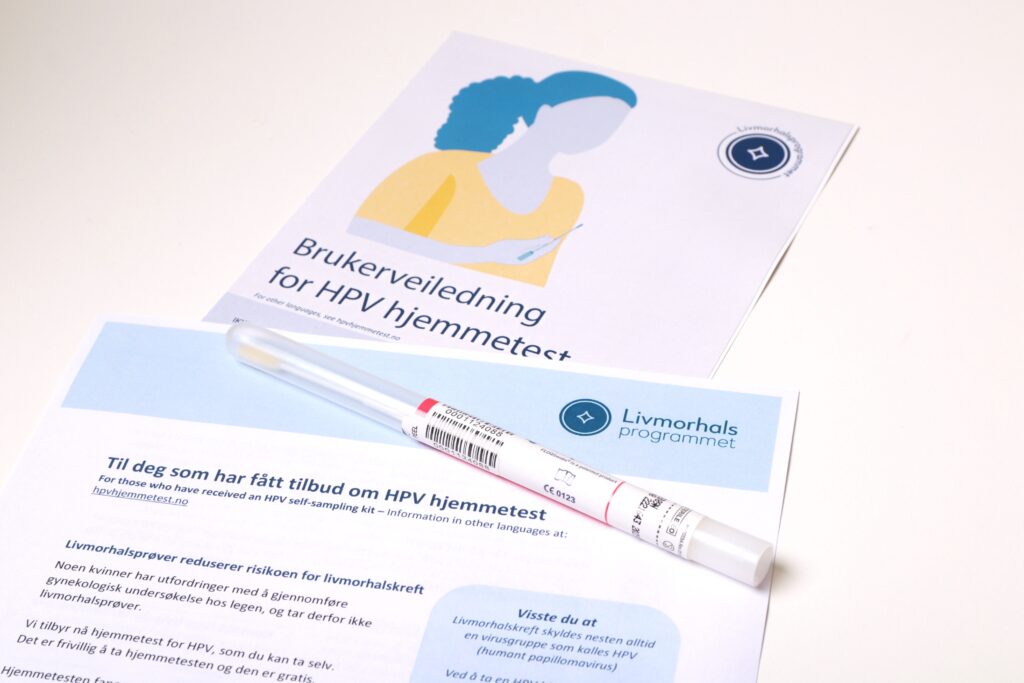
Many women avoid cervical screening due to negative past experiences, practical barriers, or a history of sexual trauma. Self-sampling at home for HPV testing helps mitigate these challenges.
The effectiveness of HPV self-sampling relies on three key factors:
- – Comparable sensitivity to physician-collected samples in detecting cervical cancer and pre-cancers.
- – Increased participation rates when self-sampling is offered compared to physician-based sampling.
- – Women follow up with further examinations after a positive self-sample.
The Cancer Registry of Norway has conducted several studies to evaluate factors affecting screening participation and the effectiveness of alternative approaches:
- – Perspectives of non-attenders: A qualitative focus group study (DOI: 1136/bmjopen-2019-029505) found that non-attenders often forget to book appointments due to life responsibilities, feel overwhelmed by arranging their own appointments, and find pelvic examinations uncomfortable. The study highlights the need for enhanced reminder strategies and the consideration of HPV self-sampling to improve participation.
- – Increased attendance with self-sampling: A 2016 study (DOI: 1371/journal.pone.0151978) demonstrated an increase in attendance among under-screened women from 23.2% to 33.4%, with 94.1% of HPV-positive participants following up with a GP.
- – Higher participation rates: Another study (DOI: 1038/s41416-022-01954-9) showed a 23% higher participation rate among long-term non-attending women who received a self-sampling kit by mail compared to those who received a regular screening invitation.
- – Effectiveness of self-sampling: A 2017 study (DOI: 1016/j.jcv.2017.12.008) demonstrated that self-sampling is as effective as GP-collected samples in detecting HPV. The study also found that self-sampling was well accepted by women and preferred over a smear collection by a medical professional during a pelvic examination.
- – Cost-effectiveness of self-sampling: A cost-effectiveness study conducted in collaboration with researchers at the University of Oslo and Harvard University (DOI: 1158/1055-9965.EPI-16-0350) suggested that targeted self-sampling for non-attenders is a cost-effective solution.
Supported by these findings, CervicalScreen Norway decided in 2023 to integrate self-sampling into the program to improve participation among long-time non-attenders.
Initially targeting women who had never been screened or had not participated in over ten years, the program will expand in 2025 to include:
- – Women overdue for screening (more than eight years since last test).
- – Women aged 29-35 who have never been screened.
Eligible women receive an invitation to collect an HPV self-sampling kit from their GP, with samples analysed at Akershus University Hospital. The program’s long-term goal is to send self-sampling kits directly to women’s homes, further reducing barriers to participation.
Ameli Tropé, the head of CervicalScreen Norway, emphasizes, “We are pleased to offer self-sampling, which lowers barriers to cervical screening. HPV self-sampling allows women to check for HPV in a private and comfortable environment. If HPV is detected, women will undergo a gynaecological examination, which can identify and potentially treat pre-cancerous changes before they develop into cancer.”

Balancing act: Ensuring equitable management for women at equal risk of cervical cancer
Commercially available HPV assays used in screening detect 14 high-risk (hr) HPV genotypes. While HPV testing is more sensitive than cytology, detecting all 14 types together does not differentiate between transient infections and persistent infections that can lead to cancer.
When HPV-based screening was implemented in 2019, all hrHPV-positive women with abnormal cytology were referred for colposcopy and biopsy, leading to a 60% increase in colposcopy referrals. This created a suboptimal clinical management system with low predictive value, excessive use of health care services, and distress among women. DOI: 10.1158/1055-9965.EPI-22-0340
In our effort to calibrate the follow-up algorithm, we relied on the premise that each of the 14 distinct hr HPV genotypes possesses its own unique carcinogenic potential and cervical cancer risk profile. More than 3000 hrHPV positive women were assessed for risk of cervical precancers and cancers while separating the most carcinogenic genotypes, HPV16 and 18, from the pool of the remaining hrHPV types. DOI: 10.1038/s41416-020-0790-1
As a result, this new screening algorithm reduced unnecessary colposcopy referrals with biopsy for women with lower precancer or cancer risk. Based on our research, the follow-up algorithm for HPV-positives was changed in July 2018.
Ongoing research aims to develop more effective clinical algorithms for managing HPV-positive women. Our focus includes methylation markers, the combination of methylation and HPV genotyping, and the integration of methylation with cytology. Several studies are also evaluating the most suitable screening strategies for HPV-vaccinated populations. Additionally, research into risk-based screening seeks to optimise cancer prevention by tailoring follow-up strategies to individual risk profiles.
Insights for European screening programs
CervicalScreen Norway demonstrates how evidence-based strategies can enhance cervical cancer screening. The integration of HPV self-sampling has improved access, particularly for underserved groups, and research suggests that direct mailing of test kits could further boost participation.
Ongoing efforts focus on optimising risk-based screening, refining triage protocols for HPV-positive individuals, and tailoring strategies for HPV-vaccinated populations. By sharing our research and experiences, we aim to support European countries in strengthening their screening programs and advancing cervical cancer prevention.
Subscribe to our newsletter to get news and updates.
Subscribe to our newsletter to get news and updates.

The general objective of EUCanScreen is to assure sustainable implementation of high-quality screening for breast, cervical and colorectal cancers, as well as implementation of the recently recommended screening programs – for lung, prostate and gastric cancers. EUCanScreen will facilitate the reduction of cancer burden and achieving equity across the EU.
This project has received funding from the European Union’s EU4HEALTH Programme under the Grant Agreement no 101162959